When Do The Macroscopic Properties Become Constant In An Equilibrium System?
When do the macroscopic properties become constant in an equilibrium system?. For this reason thermodynamics belongs to classical physics. Be notified when an answer is posted. 23 D4 Macroscopic properties become constant in an equilibrium system when A.
What happens to macroscopic properties at equilibrium. The macroscopic properties are constant d. Maximum randomness has been achieved b.
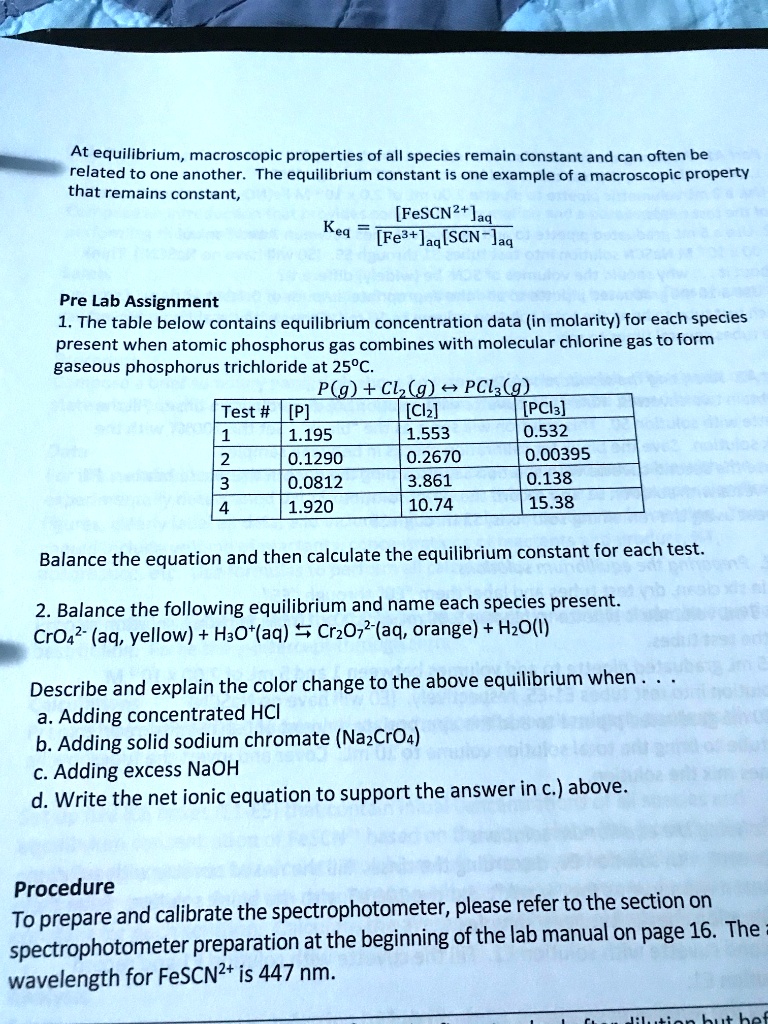
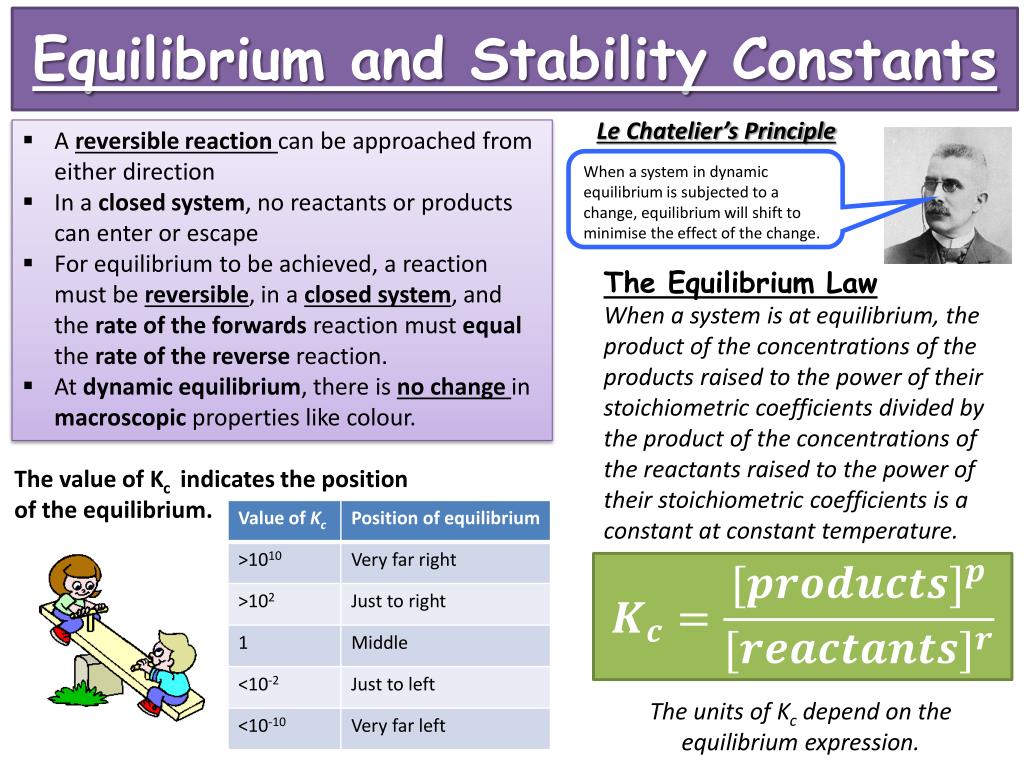
The state of the system which is in thermodynamic equilibrium is determined by intensive properties such as temperature pressure volume etc. If we run a reaction in a closed system so that the products cannot escape we often find the reaction does not give a 100 yield of products. The most common macroscopic property that stays constant is the measurement of the concentrations of substances in the reaction.
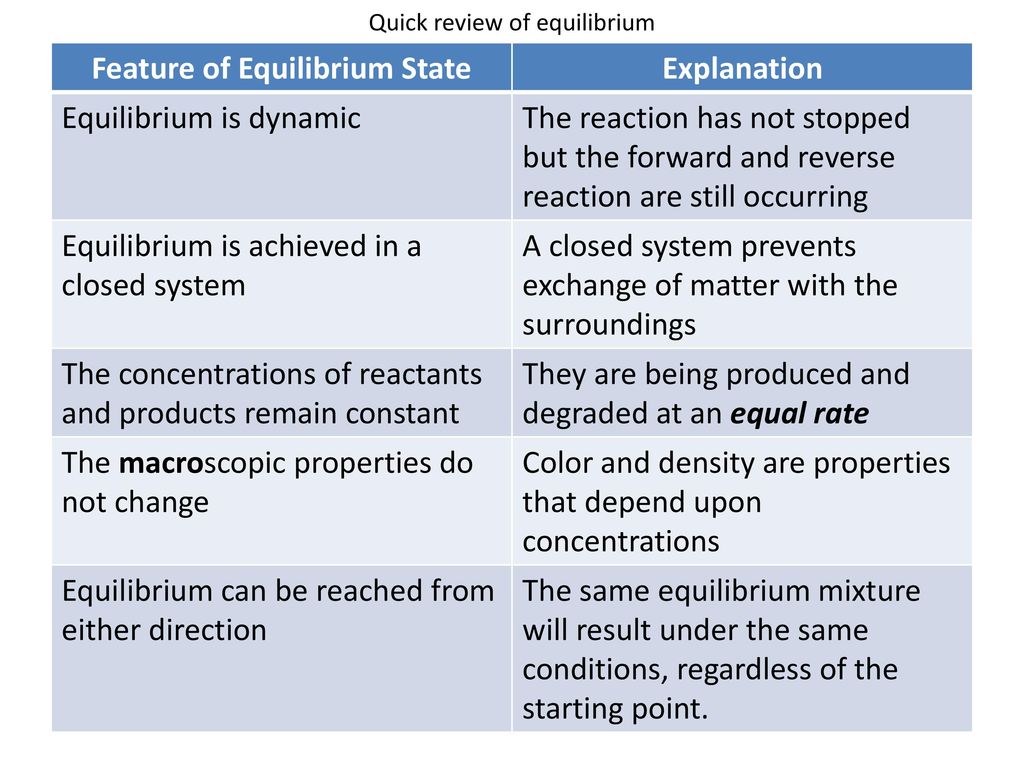
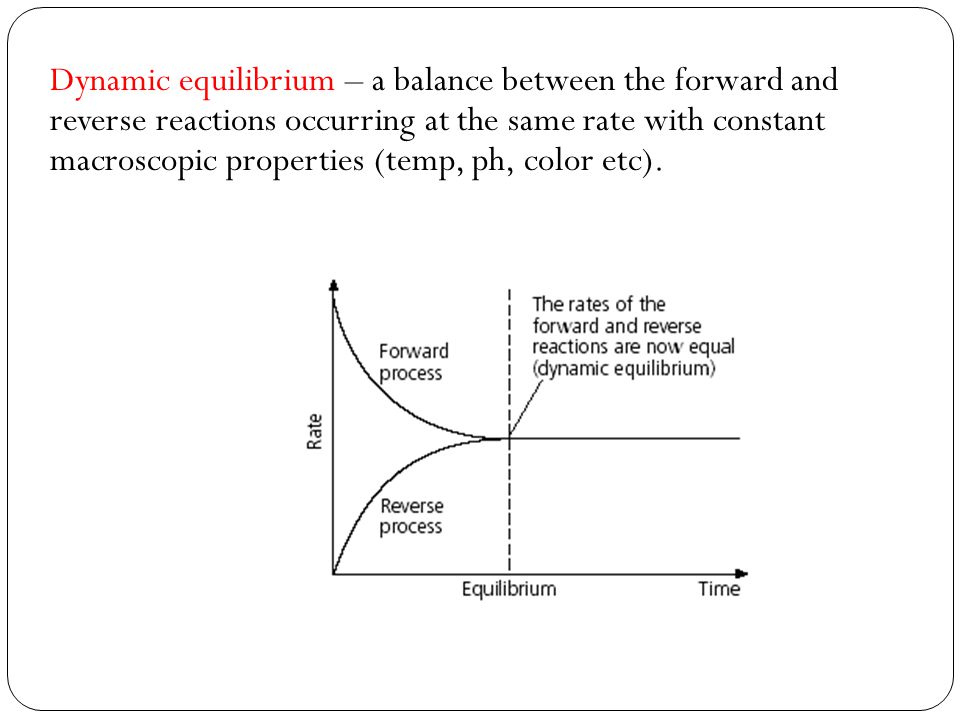
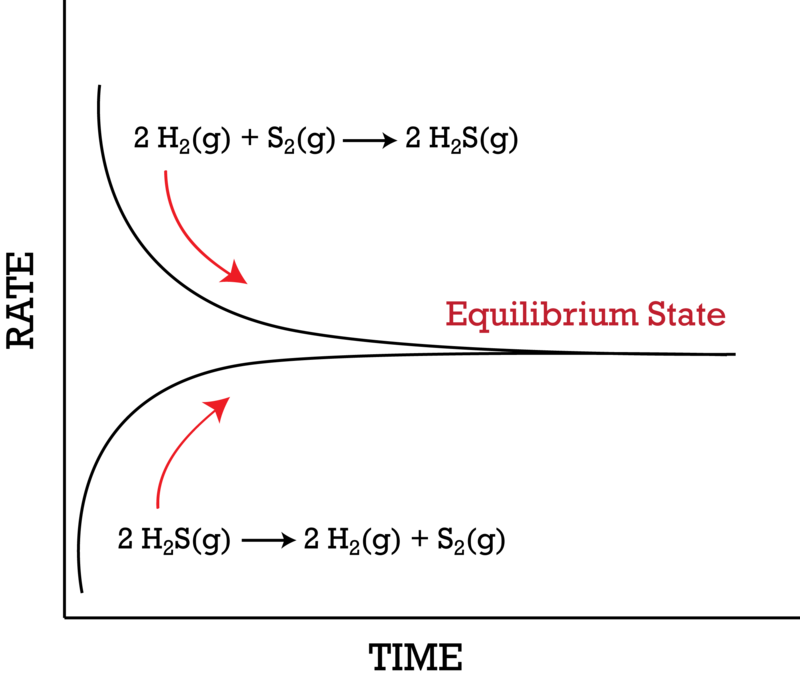
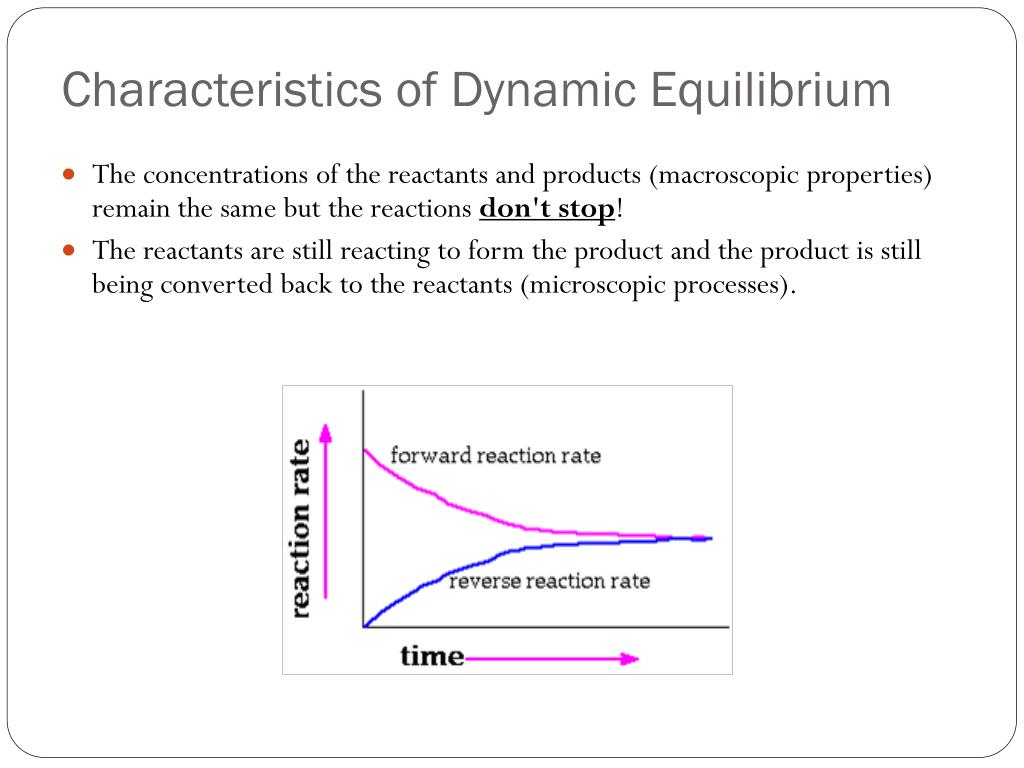
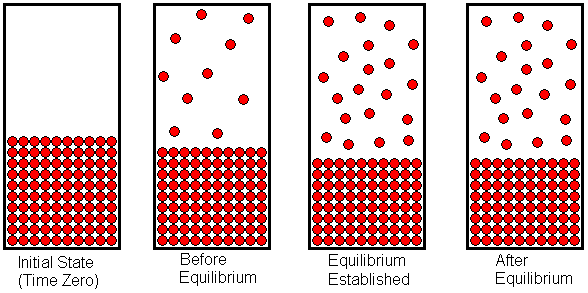
If equilibrium is established then normally chemical reaction cannot proceed in any direction at equilibrium state the amount of reactants that react to produce the product the same amount of product is converted into reactants again. A chemical equilibrium is described as dynamic because a. Forward and reverse reaction rates are equal.
As we shall see later the equilibrium state of a system is always sensitive to the temperature and often to the pressure so any changes in these variables however small will temporarily disrupt the equilibrium resulting in an observable change in the composition of the system as it moves toward its new equilibrium state. 2 on a question When do the macroscopic properties become constant in an equilibrium system. I II and III.
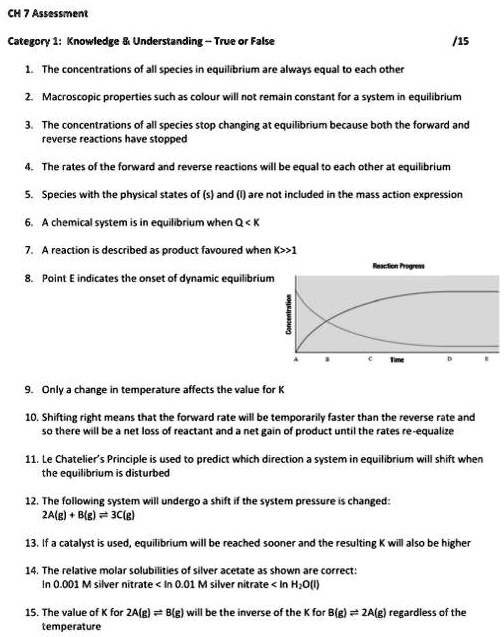
A chemical reaction is in equilibrium when there is no tendency for the quantities of reactants and products to change. Want this question answered. The reactants are completely used up.
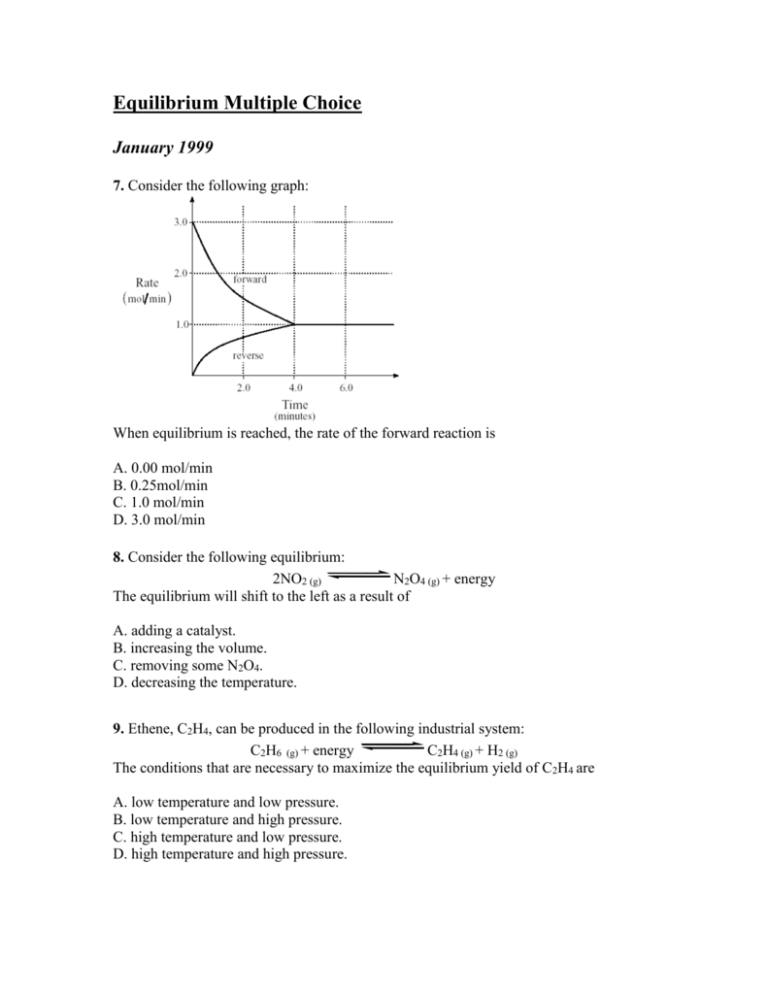
In a chemical equilibrium the forward and reverse reactions occur at equal rates and the concentrations of products and reactants remain constant. 24 D4 Which of the following does not apply to all chemical equilibrium systems.
As we shall see later the equilibrium state of a system is always sensitive to the temperature and often to the pressure so any changes in these variables however small will temporarily disrupt the equilibrium resulting in an observable change in the composition of the system as it moves toward its new equilibrium state.
All reactions have stopped. 2 on a question When do the macroscopic properties become constant in an equilibrium system. Maximum randomness has been achieved b. It is important to note that thermodynamics by itself cannot give us fine microscopic details but can only tell us about the bulk properties of the system. Thus the two equations. When do the macroscopic properties become constant in an equilibrium system. The most common macroscopic property that stays constant is the measurement of the concentrations of substances in the reaction. When forward and reverse reaction rates are. Chemistry 07122019 1431 jeniferfayzieva2018 When do the macroscopic properties become constant in an equilibrium system.
Chemistry 07122019 1431 jeniferfayzieva2018 When do the macroscopic properties become constant in an equilibrium system. When do the macroscopic properties become constant in an equilibrium system. All reactions have stopped. Macroscopic properties of materials are revealed to the metallurgist by experimental data such as stress-strain curves fatigue relaxation or creep curves which depend on external parameters temperature strain rate or loading rate and on the internal structure of the material. The pressure and temperature do not change c. If equilibrium is established then normally chemical reaction cannot proceed in any direction at equilibrium state the amount of reactants that react to produce the product the same amount of product is converted into reactants again. Thus the two equations.






























Post a Comment for "When Do The Macroscopic Properties Become Constant In An Equilibrium System?"